Recently, Prof. Wang Lianhui, Prof. Chao Jie and Associate Prof. Gao Yu, the cultivation and construction site of State Key Laboratory of Organic Electronics and Information Display and State Key Laboratory of Bio-intelligent Materials and Diagnostic and Therapeutic Technology, led the scientific research team to develop an intelligent DNA thrombolytic nanodevice. It can recognize thrombin, a biomarker of blood clots, under the complex pathophysiological environment of blood vessels, and distinguish blood clots from wound clots through logical operations on thrombin concentration, so as to realize precise drug delivery targeting blood clots. The related work was published in Nature Materials under the title of “An intelligent DNA nanodevice for precision thrombolysis”. Prof. Lianhui Wang, Prof. Jie Chao, and Associate Prof. Yu Gao were the co-corresponding authors of the paper, and PhD students Jue Yin, Siyu Wang, and Jiahui Wang were the co-first authors of the paper.
Vascular obstructive disease caused by thrombus has overtaken cancer as the most lethal disease in the world, and is the No.1 killer that endangers human beings. Thrombolytic therapy is the first choice for acute thrombosis such as stroke, heart attack, and pulmonary embolism, and thrombolytic drugs such as tissue plasminogen activator tPA can activate fibrinolytic enzymes in the body to dissolve fibrin, the main component of thrombus. However, thrombolytic drugs are a double-edged sword, over-activated fibrinolytic enzymes will indiscriminately dissolve fibrin leading to abnormal coagulation function, resulting in serious bleeding complications and life-threatening. Therefore, the realization of precise drug delivery only for thrombus is the development direction of thrombolytic therapy in the future, which will bring hope for the treatment and recovery of more thrombus patients. 1959, the famous physicist Feynman predicted “the swallowable surgeon”, envisioning the application of injectable micro-machines in medicine. DNA nanotechnology uses the four bases of the human body as the basic unit, precisely constructs functional modules and realizes nanodevices that can automatically perform a series of tasks through orderly self-assembly.
The team's researchers first constructed 90 x 60 nm rectangular nanosheets based on DNA origami technology and achieved precise and controllable spatial and quantitative loading of the thrombolytic drug tPA molecule on its surface. Subsequently, utilizing the DNA triple-stranded structure, logically operable DNA nanolocks were designed to curl the rectangular nanosheets shut to form nanotubes to protect the drug activity. The DNA nanolock containing thrombin aptamer is the computational core of the thrombolytic nanodevice, which automatically performs a series of tasks of tracking recognition, logic operation and response opening against thrombin in a set sequence. In response to the difference in thrombin concentration between thrombus and wound clot, the DNA thrombolysis nanodevice equipped with a specific thrombin concentration threshold controller realizes the logic operation for thrombin concentration for the first time in animals. It can accurately distinguish between normal fibrin clot and thrombus, significantly reduce the coagulation abnormality caused by thrombolytic drugs, and realize intelligent and precise drug delivery, which has been verified in animal models of stroke, pulmonary embolism, carotid artery and leg vein thrombosis.
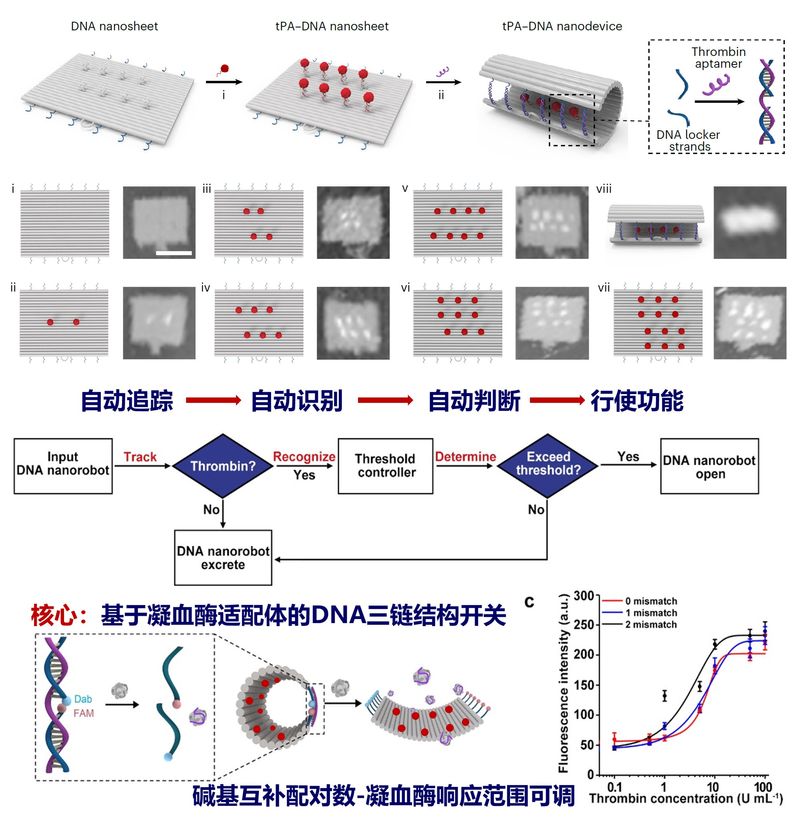
The Intelligent DNA Thrombolytic Nanodevice Based on DNA Nanotechnology
Compared with the clinical thrombolytic drug tPA, the intelligent DNA thrombolytic nanodevice has improved the thrombolytic efficiency in stroke and pulmonary embolism by 3.7 times and 2.1 times, respectively; the complete thrombolytic dose has been reduced by 6 times compared with that of tPA; and the coagulation abnormality caused by clinical thrombolytic drugs has been significantly reduced, thus extending the window of treatment for stroke from 3 hours to 6 hours after the onset of symptoms, which is expected to significantly increase the number of stroke patients receiving thrombolytic therapy and benefiting from it. The DNA thrombolytic nanodevice is composed of human bases, which can be degraded by enzymes and metabolized by the liver and kidneys, and therefore has excellent biocompatibility and good prospects for clinical translation. The research team plans to complete the efficacy and safety evaluation of the smart DNA thrombolytic nanodevice in large animal models, the study of the drugability of the DNA thrombolytic nanodevice, and the optimization of the scale-up production process in the next 3-5 years, and then actively report to the clinical trials and strive to obtain the approval for the clinical trials.
The project was supported by National Center for Basic Science, National Natural Science Foundation of China and Jiangsu Province Frontier Leading Technology Basic Research Special Project.
Original Text: https://www.nature.com/articles/s41563-024-01826-y
(Written by Gao yu Initially Reviewed by Gao Zhihua, Ling Haifeng Edited by Wang Cunhong Reviewed by Zhang Feng)



